|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
Discussion Table 3. The average number of beats per
minute in each solution produced by all the hearts (groups A
and B). The average bpm of groups A and B combined in each
solution are also shown below as a percentage of the base
rate. Average Number of bpm Average % of BR DMEM (Base Rate) 0.1 mg/ml Caffeine 0.2 mg/ml Caffeine 0.3 mg/ml Caffeine 0.5 mg/ml Caffeine 1.0 mg/ml Caffeine
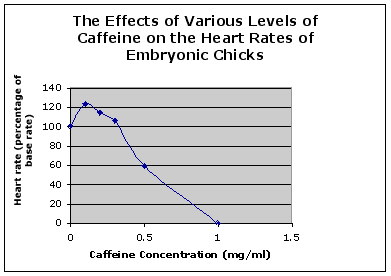 Figure 11. The relationship between the caffeine concentration and the heart rate as a percentage of the base rate. The mark at zero caffeine represents the base rate in the DMEM control.
In the preceding experiment on this topic, the heart was removed from the embryo to observe the effects of caffeine on the heart rate placed in DMEM and various concentrations of caffeine. They have found that the heart rate decreased as their caffeine concentration increased. We decided to keep the heart intact with the embryo to see if the extraction cause distress on the embryo causing the hear t rate to drop. Our result refutes the previous findings and confirmed our original hypothesis (Table 3 & 4). The sodium and calcium gradient or current regulates the rate and function of the heart. As more sodium ions enter the heart, the faster the heart will beat. Caffeine increases the current carrying theses positive sodium ions. Therefore, as the concentration of caffeine is increased in the cells, the beats per minute will increase. This increase will only occur until the cells can no longer withstand the induced stress of the current. Our results show that as caffeine was introduced to the embryos, a clear difference was seen in the heart rate of each individual embryo and at different concentrations. As the concentration of caffeine increased, the average beats per minute (bpm) also increased. At .25 mg/ml of caffeine the average change in heart rate from the control (Howard's Ringers) was 2 bpm, 8.5 bpm at a caffeine concentration at .5 mg/ml, and lastly and increase of 10 bpm at a caffeine concentration at 1.0 mg/ml. In previous experiments, DMEM and Howard's Ringers was used for the control. We only used the Howard's Ringers because research data has shown that the addition of glucose (DMEM) is unnecessary for intact embryo. In a study run at the University of Wisconsin, the effects of caffeine were measured on three day old chick embryos. It was concluded that caffeine resulted in cardiac malformations as well as yielding toxic effects of the cells (Bruyere 1986). Also in this experiment, the stroke volume and cardiac output both increased with the concentration of caffeine. This shows how caffeine is able to increase the flow of sodium and calcium ions into the cardiac cells increasing and stimulating cells and heart. This conclusion is also supported by our study. Consumption of different concentrations of caffeine has various effects on humans. Caffeine (1,3-trimethylxanthine) is a natural alkaloid found in coffee beans, tea leaves, cocoa beans, cola nuts and other plants (Nawrot, et. al 2002). Coffee is probably the largest source of caffeine intake for humans. For those who consume less than four cups (<400mg) of coffee per day does not affect the cardiovascular health adversely.Coronary heart disease is associated with the utilization of 10 or more cups (> 1000mg) of coffee per day. There has been some debate on whether pregnant woman the consumption of coffee is risky for the fetus.Caffeine is a drug that readily crosses the placental barrier. Studies have shown that during the third trimester, caffeine intake between 71 to 140 mg per day resulted in low birth weight. Pregnant women who smoke and consume caffeine (>300mg/day) has a greater risk for a low birth weight. A high dose of caffeine intake may also lead to intrauterine growth retardation. Further experiments may be conducted to observe the affect of caffeine on the overall development of chick embryo to see if it actually causes a low birth weight. In the future, we wish to increase the sample size of chick embryos for the experiment in order to have a greater variance in the results. Also, it may benefit to run trials on multiple concentrations of caffeine such as 1.5 mg/ml, 3.0 mg/ml, and 4.5 mg/ml to observe the concentration where it becomes damaging to cell and cause cessation.
|
||||||||||||||||||||||||
|
|
Bellains, Ruth. Osmond, Mark. 1998. The Atlas of Chick Development. Academic Press, San Diego. pp. 25-29. Braun, Stephen. 1996. Buzz: The Science and Lore of Alcohol and Caffeine. Oxford University Press, New York. pp 155-156. Bruyere, Harold J., Bernard J. Michaud, Enid F. Gilbert, and John D. Folts. "The Effects of Cardioteratogenic Doses of Caffeine on Cardiac Function in the 3-day Chick Embryo." Journal of Applied Toxicology 7.3 (1987): 197-203. Print. Gilbert, Scott F., 2010. Developmental Biology, 9th ed. Sinauer Associates Inc., Sunderland, Mass. Gwinn, Robert P. 1992. The New Encyclopedia Britannica. Encyclopedia Britannica, Inc., New York. pp. 384-390 Lee, H., R. G. Nagele, and J. F. Pietrolungo. "Toxic and Teratologic Effects of Caffeine on Explanted Early Chick Embryos." Teratology 25.1 (1982): 19-25. Print. Narwot, P. "Effects of Caffeine on Human Health." Food Additives and Contaminants 20.1 (2003). Print. Nishikawa, T. et al. 1985."Potentiating Effects of Caffeine on the Cardiovascular Teratogenicity of Ephedrine In Chick Embryos." Toxicology Letters. 29: (1985) 65-68. Spiller, Gene A. 1998. Caffeine. CRC Press, New York. pp. 228-229. |
|||||||||||||||||||||||||