|
Procedure:
1.Cut
small pieces of Whatmann 3MM filter paper (3mm X 3mm) and
soak them with 3 ml Hydrocortison-acetate (3 mg/ml in
ethanol) (Sigma). Air-dry the filter discs for 1.5 hrs.
2.Obtain
4mL each: bFGF (1.5 ug/ml), VEGF
(1.5 ug/ml), or DMEM (a tissue
culture medium in which the control filter discs should be
soaked). Pour the solutions into separate petri dishes that
are labeled with the names of the compounds. Soak the filter
paper disks in the solutions for 30 minutes.
3.While
the filter paper is soaking, obtain ten-day-old chick eggs
and wash them in 70% ethanol. Label the eggs as either DMEM,
bFGF, or VEGF using a pencil. Open the blunt end of the egg
with forceps. Peel back the shell membrane, being careful
not to damage the CAM membrane.

4.Drop
the filter paper over the embryo in the area with the least
number of visible blood vessels. Cover the hole in the
shells with Scotch tape, and place in an incubator set at
37C (being careful not to jostle the embryos) for four
days.
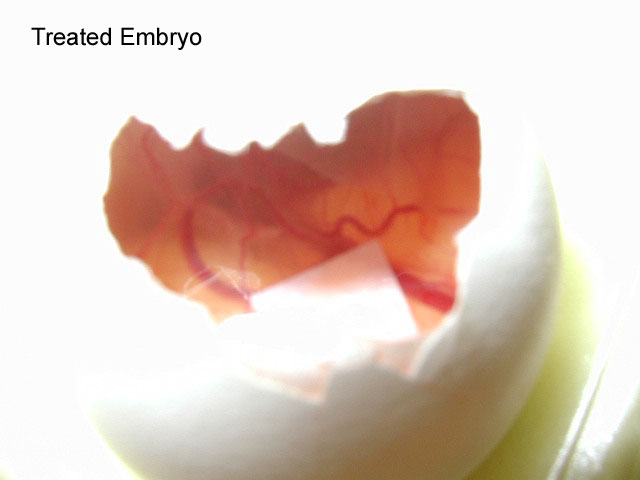

5.After
the four-day period, remove the tape covering the holes and
extract the filter paper using a pair of forceps. Examine
the filter paper for the presence of blood vessels. Count
all blood vessels on the filter paper and record the numbers
within your lab notebook. Compare to the other
conditions.
Instructor's
prep sheet
Lab
Protocol
|
