
Can testosterone make your chick masculine?
Rachael Curley, Zac Martin, Michael Mendez
Milersville University
Purpose
To determine if the sex of chicken
embryos will be influenced by the injections of hormones, particularly
testosterone. Testosterone is a cholesterol-derived hormone that is found at
higher concentrations in males than females. The desired results following the
testosterone injections are to obtain all male, female and transgender
containing ovary and testis, chick embryos. After the dissection of these
chicks, the gonads will be stained to help distinguish the organism between
male and female as described in our previous study (chick mini-lab).
Background
Chicken embryos
begin to develop their sex organs around 7 days following fertilization. During
this crucial developmental stage the left gonad of both male and female
increase in volume. The difference is greater in females than in males,
constituting early evidence of some sex differentiation of the gonads.
Additionally, volume and surface epithelium thickness are greater in the left
gonad in both sexes. These differences disappear in males, whereas in females
only the left ovary and left Mllerian ducts fully develop and become
functional (Hoshino, 2005). It has
been previously hypothesized that sex hormones, when increased in
concentration, may influence the development of gonads and in fact cause
reveral sex development. Theelin, theelol, and an extract of male human urine
(containing some estrogenic substances) brought about, in a number of cases,
embryos in which the left gonad is an ovotestis and the right one entirely
testicular (Willier, 1937).
Chicken embryos have distinct
differences between their reproductive organs. Males have two testis and two
ovaries form in the female, but then only one continues to grow and develop. It
is very hard to distinguish between both, when just viewing the gonads under a
microscope. Therefore, a staining technique using alkaline phosphatase
substrate is implemented since germ cells can be stained based on their high
levels of the enzyme alkaline phosphatase. Staining the primordial germ cells
(PGCs) would allow you to distinguish between ovaries and testis in younger
chicks because they still have two ovaries and the shapes are more similar. Alkaline
phosphatase activity was first noted with consistency in PGCs as early as 2
days of incubation. PGCs found in the extra embryonic circulation at this stage
of development exhibited a positive reaction for alkaline phosphatase activity
in the form of a globular deposit(s) in the cytoplasm near the nucleus.
(Swartz, 1982) According to Gilbert (2009), PGCs are arranged differently in
testis and ovaries. In the testis the germ cells migrate to the periphery of
the seminiferous tubules where they begin to differentiate into sperm and then
are transported through the rete testis. In the females the germ cells become
the ova and the surrounding cortical somatic cells will differentiate into
granulose cells. From there the mesenchyme cells from the ovary differentiate
into the thecal cells.
Materials:
24-two week old embryos
HowardÕs Ringers Solution
Dissection tools (2x)
Petri-Dishes (1 per 2 Chicks)
Scanning Microscope
Alkaline Phosphatase Substrate (Western Blue, Fisher)
Alkaline Phosphatase Substrate Buffer (APSB)
1x PBS (Phosphate
Buffered Saline) Stain Stop
4% PFA
(PARAFORMALDEHYDE) in PBS
Capped Tubes (8 tubes)
Eye droppers (2x)
Testosterone
Cypionate
1mL Syringe
Vegetable Oil
Beak nose tweezers
Scotch Tape
Procedure:
Making Solutions
APSB
(100nN NaCl, 100mM Tris, pH 9.5,
50mMMgCl2)
5 M NaCl 2ml
2
M Tris, pH 9.5 5ml
4.9
M MgCl2 1ml
dH2O
(ultrapure) to 100ml
1x PBS
10X PBS
NaCl 175.3g
KH2PO4 7.7g
K2HPO4
(anhydrous) 25g
dH2O to
2L
100mL of this solution was added to
900mL of H2O to create 1L of 1xPBS
APS
Western Blue
Cat # S3841 Lot # 28564204
4% PFA
PBS
– Warm to 65” (about 1 hour)
Add
4g/100ml Paraformaldehyde
Add
1 ml 1M NaOH, stir till dissolves
Neutralize
with 1 ml of 1M HCl
Testosterone
Cypionate/Vegetable Oil
The Testosterone Cypionate (TC)
bottle recommended 400mg TC per 100kg body weight. This was converted to 50µg TC 10g body weight. A random sample of five eggs were weighed
and averaged to be 66.576g. The
weight was rounded up to 70g so the calculated dosage would be 350µg of TC per
70g of body weight. The TC bottle
contained 10mL of solution with a dosage of 100mg/mL. We calculated that each egg should receive 3.5µL of TC. The final solution contained 105µL of
TC solution mixed with 2.9mL of vegetable oil to give us a total of 3mL. This is the solution used for
injection.
Testosterone Injection
Allow the eggs to sit up right for 5 min in order to ensure
that the chick embryo is at the top of the egg. Using the beak nose tweezers, poke a small hole in the shell
of the egg allowing enough room to see where you are going to stick the
needle. Keep in mind you are not
to poke the embryo itself with the needle. Using a 1mL syringe, measure out 100µL of Testosterone
Cypionate/Vegetable Oil solution.
Inject this into the yolk of the egg through the hole. Immediately after injecting the
Testosterone Cypionate/Vegetable Oil solution into the yolk, place a piece of
scotch tape over the hole to prevent the embryo from drying out.
Staining Process
Fertilized
chicken eggs were obtained from Millersville University of Pennsylvania where
they were incubated at 37”C. To
insure the eggs were actually fertilized or developing, each egg was held up
against a table light for the presence of blood vessels lining the interior of
the egg. The eggs that did not fertilize or develop correctly were discarded.
The eggs however that did develop normally were cracked open. Using forceps the
chick embryos were pulled out and placed into a Petri dish containing 50 mL of
Howards Ringers Solution. After the chicks were decapitated and then placed on
its back, the ventral side was cut open and the stomach, intestinal tract
(white), and the liver (green/brown) were removed or pushed aside. Once removed,
the kidneys (yellow) were visible. The gonads were located towards the
medial-anterior ends of the kidneys, depending on how far along the developing
female chick embryosÕ reproductive system was. The gonads were carefully teased
away from the kidneys and then were placed into a 15 mL capped tube filled with
4% paraformalaldehyde (PFA). From there, the gonads were washed three times at
5 minute increments with another 15 ml of 4% PFA and then transferred into
another capped tube containing alkaline phosphatase substrate buffer (APSB) for
30 minutes on a rocker. After 30 minutes, the gonads were then transferred to
another capped tube filled with 5 mL APSB and 5 mL APB in a 1:1 ratio,
respectively. The gonads were
removed from the stain once the inside of the gonads turned purple and from
there, were placed in 15 mL of 1% phosphate buffered saline (PBS) for 5 minutes
to stop the precipitation. Lastly, the gonads were stored in the 15 mL of
fixative. The differences between the male and female gonads were analyzed
using a dissecting microscope.
Results
Following the injection of
testosterone into the albumin of 42 individual chick eggs albumin and the
incubation at 37”C for 7 days, the gonads were extracted. Each individual was
observed using a dissecting microscope and then stained. After the staining of
the primordial cells located inside the chick embryos gonads, a purple
precipitate was deposited. The purple precipitate enabled us to view the
make-up of the gonads. The female gonads (Figure 1) did not have a particular
pattern to them, but seemed to have random spots of purple scattered all over
the gonad(s). Female gonads are also much larger and most of them were more
round in shape. The male gonads (Figure 2) had a pattern of purple to them,
very similar to the human maleÕs seminiferous tubules pattern. Male gonads also
tended to be longer and much thinner than the ovaries. Table 1 represent the
percentage of males vs. females found in the total eggs and used as the
control. A total of 36 eggs were obtained and 23 of these eggs were actually
used for gonad extraction. Out of the eggs, 47.83% of them were males and
52.17% of them females. Table 2 presents the gender of the chicken embryos
following testosterone injections. Only 18 chicken embryos survived the
injection of testosterone and of these 61.1% were males and 38.9% transgender.
There were no females distinguished using the dissecting microscope and
staining technique. As you can see from the control to the testosterone
injected embryos there was a significant decrease in the females developed and
an increase in the males developed. From the testosterone injected embryos
there were transgenders that developed containing both an ovary and
testis. Of the 42 chicken embryos
injected only 18 developed successfully (42.85%). The high rate of
non-developing embryos following injection could be related to infection after
cracking open the eggs or from accidentally injecting the testosterone directly
into the embryos. Lastly, figure 3 represents a transgender chick embryo
following testosterone solution injection of 1 ml.
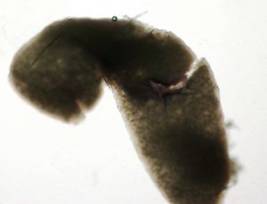
Figure 1: The ovary
of a 14-day-old chick embryo that has been stained with alkaline phosphatase
substrate (5 mL) and alkaline phosphatase substrate buffer (5 mL) in a 1:1
ratio, respectively.

Figure 2: The testis
of a 14 day old chick embryo that has been stained with alkaline phosphatase
substrate (5 mL) and alkaline phosphatase substrate buffer (5 mL) in a 1:1
ratio, respectively.
|
|
Total |
#
Male |
%
Male |
#
Female |
%
Female |
|
Exp. 1 |
15 |
8 |
.53 |
7 |
.47 |
|
Exp. 2 |
8 |
3 |
.38 |
5 |
.62 |
|
Total |
23 |
11 |
.48 |
12 |
.52 |
Table 1: The
total number of eggs that were used to extract the embryo gonads are
represented here. The total is the amount of these eggs that were developed.
The number of male and female embryos was recorded and the percentage of each
gender calculated.
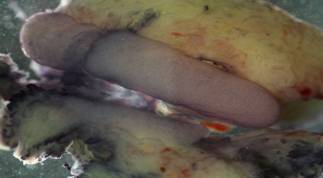
Figure 3: The gonads of a
transgender are shown. The larger pink medial object is an ovary. The smaller
pink medial object below the ovary is a testis.
|
|
Total |
# Male |
% Male |
# Female |
% Female |
# Transgender |
% Transgender |
|
1 |
11 |
8 |
.727 |
0 |
0 |
3 |
.272 |
|
2 |
7 |
3 |
.429 |
0 |
0 |
4 |
.571 |
|
Total |
18 |
11 |
.611 |
0 |
0 |
7 |
.389 |
Table 2: The
total number of eggs that were used to extract the embryo gonads are
represented here. The total is the amount of these eggs that developed
following testosterone injections. The number of male, female, and transgender
were recorded and the percentage of each gender calculated.
Discussion
This experiment achieved the ability to influence the sex of
7-day-old developing chick embryos by injecting higher amounts of testosterone
into the albumin of the egg. This
created a higher concentration of testosterone than normally present. The gonads of the chick embryo were
removed at 14 days and observed.
We also learned that alkaline substrate phosphatase, when used in the
correct concentrations, can stain the PGCs of the gonads as well as other
structures. We were able to successfully isolate the gonads of both genders as
well as stain the PGCs present in the gonads of chick embryos to assist in the
distinguishing of their gender.
All of the surviving chicks were either male or transgender following
testosterone injections. No females
were present after the injection of testosterone. This experiment shows that the sex of developing chick
embryos can be influenced using testosterone. Male or transgender chicks may be obtained by simply
injecting the eggs with testosterone.
This may be used in a practical sense on farms or other chicken
producing industries. If a higher
frequency of males is needed for reproduction of chickens then the handler of
the chickens can perform the injection of testosterone to achieve his desired
frequency. A way to expand on this
experiment would be to inject chicken embryos with Estrogen, the female sex
hormone. The same steps can be
taken to see if the sex of chickens can be influenced to be female. This would allow for a wider range of
achieving desired sex ratios. The
staining process can be used to determine the structure or organs containing
PGCs and also can be used to follow the migration patterns of PGCs through
stages of development.
Literature Cited
Gilbert, Scott F. Developmental Biology. Sunderland, Mass.: Sinauer Associates, 2006. Print.
Hoshino, A., Koide, M., Ono, T. and Yasugi, S. (2005).
Sex-specific and left-right asymmetric expression pattern of Bmp7 in the gonad
of normal and sex-reversed chicken embryos. Dev. Growth Differ 2005, 47:65 -74.
Smith, C. A. et al. Cloning and expression of R-Spondin1 in
different vertebrates suggests a conserved role in ovarian development. BMC
Dev. Biol. 8, 72 (2008).
Swartz WJ. Acid and Alkaline
Phosphatase Activity in Migrating Primordial Germ Cells of the Early Chick Embryo. Anat Rec. 1982; 202:379–385.
Tsiper SM. The Alkaline Phosphatase Activity of the Tissues
and the Primordial Germ Cells during the Embryonic Development of the Mouse. Bulletin of Experimental Biology and Medicine. 1958; 1271-1274.
Willier, B. H.; Gallagher, T.
F.; and Koch, F. C. 1937. The modification of sex development in the chick embryo
by male and female sex hormones. Physiol. Zoo. 6l:101-22.
Yuankai,
T. Bridging Evolutionary Gaps: Unearthed dinosaur fossils shed light on the
evolutionary process. Beijing Review (2009): 22-23. Web. 26 April 2010.