|
Concentration-dependent Effect of Nicotine on Polyspermy Induction in Sea Urchin Fertilization
Timothy Dao and Rob Hyson
Millersville University
Introduction:
A detractor of external fertilization, a common mode of reproduction in marine organisms, is the threat of polyspermy. Any egg that fails to prevent multiple sperm from fertilizing it will not produce a viable embryo. Researchers such as Longo (1970), and Jaffe (1980) have looked into the methods sea urchin eggs, Lytechinus variegatus, employ to prevent polyspermy. The cortical reaction, also referred to as the slow block (Jaffe, 1986), directly affects development of the vitelline envelope, a permanent barrier against additional sperm. The cortical reaction is electrically stimulated through the influx of Ca2+ into the egg and causes the release of cortical granules, which unleashes enzymes to detach the vitelline membrane as well as destroy bindin/sperm receptors on the egg's plasma membrane (Jaffe, 1986). While this is not the sole mechanism for preventing polyspermic fertilization, it lasts significantly longer than Na+ fast block and creates a physical barrier against sperm entry. The cortical reaction is what we decided to focus on in our experiment. Nicotine is a known Ca2+ channel blocker (Jaffe, 1980). With it, we hoped to block the cortical reaction and create a polyspermic environment for the eggs.
Objective:
Nicotine is already a known polyspermic agent (Jaffe, 1980; Jaffe, 1906; Longo, 1970), so our experiment is not ground breaking. Nonetheless, the primary focus of this experiment is to discover what concentration of nicotine would be needed before the cortical reaction becomes completely disabled resulting in polyspermy of all eggs. Odd cleavage planes or eggs that divide but do not have an obviously deployed vitelline envelope would be clear indicators of polyspermic eggs. Research by Longo (1970) indicates that it should take relatively little nicotine to induce at least 98% rates of polyspermy. We hoped to map which concentrations are most effective in stopping the cortical reaction by exposing different groups of eggs to various concentrations of nicotine. We hypothesize that as the nicotine concentration increases, the rate of polyspermic fertilization should increase until it reaches 100%. The plotted curve should be relatively S-shaped as there is an upper limit of 100% polyspermy. Note that we were not able to predict where the point of diminishing effectiveness would be at since all the research measured nicotine concentration by percent instead of molarity.
Materials:
á ASW (Artificial Sea Water)
á 20 test tubes
á Micropipette and disposable caps
á Disposable pipettes
á Sigma Aldrich 40% concentrated Nicotine Solution
á Well Slides and cover slips
á Newly collected urchin (Lytechinus variegatus) eggs and sperm samples
á Glove and protective eye wear
á Stereomicroscope
Procedure:
1. Collect Urchin eggs and sperm, keeping them separate.
2. Wash the eggs two times with ASW (artificial salt water) as preparation.
3. Divide the prepared eggs with a pipette into seven separate test tubes, each test tube with approximately 1 ml of ASW. The tip of the pipette may be cut off to ensure that the eggs are not crushed while moving them about. Also, be sure to keep the pipettes separate from each other for the rest of the experiment to prevent accidental mixing of solutions and eggs.
4. Label each of the test tubes 1-7 using tape or a marker
5. To prepare the nicotine solution: obtain a test tube with 10 ml of ASW and add 1 µl of 40% concentrated nicotine to it with a micropipette. Be sure to be wearing gloves and goggles as concentrated nicotine is very poisonous neurotoxin
6. Mix the nicotine base solution. It should have a nicotine concentration of 1 mM. set aside to add to the experimental test tubes
7. The following table shows the contents of each test tube
|
Test Tube # |
Egg containing ASW solution (ml) |
Pure ASW (ml) |
Prepared nicotine Solution (ml) |
Total Volume |
Nicotine Concentration (mM) |
|
1 |
1 |
3 |
0 |
4 |
0 |
|
2 |
1 |
2.5 |
0.5 |
4 |
0.125 |
|
3 |
1 |
2 |
1 |
4 |
0.25 |
|
4 |
1 |
1.5 |
1.5 |
4 |
0.375 |
|
5 |
1 |
1 |
2 |
4 |
0.5 |
|
6 |
1 |
0.5 |
2.5 |
4 |
0.625 |
|
7 |
1 |
0 |
3 |
4 |
0.75 |
8. Then, test tube 1 receives 3 ml of ASW, no nicotine this is the control group.
9. Test Tube 2 gets 2.5 ml of ASW and 0.5 ml of nicotine, making the nicotine concentration is 0.125 mM.
10. Test tube 3 receives 2 ml of ASW and 1 ml of nicotine, making the nicotine concentration 0.250 mM.
11. Test tube 4 receives 1.5 ml of ASW and 1.5 ml of nicotine with its concentration being 0.375 mM.
12. Tube 5 receives 1 ml of ASW and 2 ml of nicotine, its final concentration is 0.5 mM.
13. Tube 6 receives 0.5 ml of ASW and 2.5 ml of nicotine making its concentration 0.625 mM.
14. Finally, tube 7 receives 3 ml of nicotine making its final concentration 0.750 mM.
15. Once the tubes have been set with the eggs and nicotine solution, Check the sperm for motility in the sperm sample using a microscope.
16. Add 2 drops of activated sperm from a pipette to each test tube and gently mix the contents of the tubes. This should only add .2 ml of ASW and should not impact the concentration of nicotine drastically
17. Wait approximately 90 minutes or until the eggs begin to divide.
18. Take a small sample of eggs from each test tube and place into separate well slides.
19. Take count of the number of typically dividing eggs and take a count of the eggs that exhibit polyspermy. Also, for correct scaling, count the number of unfertilized eggs
Procedural Notes:
Several aspects did not go according to plan during our experiment. We wanted to use the sperm immediately after we finished setting up the test tubes. However, time was not on our side and we were forced to let the eggs sit in the nicotine solution for approximately an hour before the sperm sample was ready for use. This may possibly have killed the eggs before we could add sperm to them and can possibly throw all our results off. We assumed that the eggs were still be viable and proceeded under that assumption. Further research must be done, or this experiment needs to be replicated in order to have more conclusive and certain results.
Results:
Each sample of eggs we took from each group contained an average of 34 eggs. The eggs were easily visible under the stereomicroscope with minimal magnification. The control group exhibited no signs of polyspermy. However, half of the eggs did not begin cleavage at 90 minutes after the introduction of sperm. Eggs that did not begin cleavage within 90 minutes after the introduction of sperm were regarded as unfertilized. As seen in the base control group, there was a relatively low rate of fertilization with our sample of activated sperm. The experimental groups did not fair any better, with half or more of the eggs remaining unfertilized. At higher concentrations of nicotine, above 0.500 mM, no fertilization occurred as our samples failed to begin division after 90 minutes of waiting. The test tubes containing nicotine between the ranges of 0.125-0.375 mM, inclusive, did have eggs with multiple irregular cleavage planes (evidence of polyspermy). The 0.250 mM nicotine experimental group had the highest number of eggs exhibiting signs of polyspermy; however, as the concentration of nicotine increased, the number of eggs fertilized dwindles down to zero.
Table 2.
|
Nicotine Concentration (mM) |
# of nondividng Eggs |
# with Normal Cleavage |
# exhibiting ployspermy |
Total Count |
|
|
1 |
(Control) 0.000 |
15 |
17 |
0 |
32 |
|
2 |
0.125 |
19 |
14 |
1 |
34 |
|
3 |
0.250 |
19 |
7 |
11 |
37 |
|
4 |
0.375 |
26 |
1 |
5 |
32 |
|
5 |
0.500 |
31 |
0 |
0 |
31 |
|
6 |
0.625 |
24 |
0 |
0 |
24 |
|
7 |
0.750 |
39 |
0 |
0 |
39 |
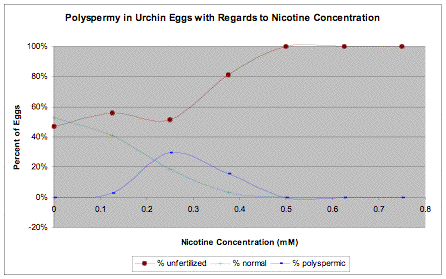
Figure 1.
Discussion:
There are several trends to note. Firstly, increasing concentration of nicotine does allow polyspermy to occur, but only to a point. At the lower concentrations, polyspermy did occur with the maximal number of polyspermic eggs appearing at the concentration of 0.250 mM nicotine. 0.250 mM seems to be the most effective concentration to use that would disable the cortical reaction, to allow polyspermy, without drastically reducing fertilization rates. Any concentrations above 0.375 mM however had no polyspermic eggs.
As the concentration of nicotine increased, the number of successful fertilization decreases. We believe that introducing the sperm into a nicotine containing solution resulted in this trend. In the experimental groups with concentrations of nicotine over 0.375 mM, all fertilization seems to have been completely prevented. Our hypothesis did not predict this effect, which was more of an oversight while we were designing our experiment. Research by Longo (1970) indicates that spermatozoa do not react well to nicotine containing solutions. Sperm motility degrades within 5 minutes of contact with a nicotine containing solution. The completely lack of fertilization with the experimental groups indicates that concentrations of nicotine above 0.375 mM completely disables the sperm. A way to avoid disabling sperm would be to wash off the eggs after they have been exposed to the nicotine solution and replace the solution with standard ASW. This would prevent nicotine from disabling the sperm. However, we do not know if nicotine would have permanently disabled the cortical reaction; washing the eggs might restore their ability slow block.
Conclusion:
We will have to void our original hypothesis. We did not receive the S-curve that we were expecting, and instead got a bell-like curve where both high and low concentrations of nicotine have a negative impact on the rate of polyspermic fertilization. We have confirmed in our experiment that nicotine is a spermicide and will prevent fertilization when at a high enough concentration; all of this is under the assumption that the eggs were still viable after sitting in nicotine containing solution. However, this result may still be uncertain due to several mistakes made during the planning stages and the way we had to perform the experiment due to the lack of materials at the needed time. We did not read though our material thoroughly enough to realize what detrimental effect nicotine would have upon the sperm we used. However, due to our inability to decide whether nicotine's effects were permanent lead us to the decision for allowing fertilization to occur within the nicotine solution rather than cleaning the eggs again and then fertilize them in ASW. In addition, our inability to obtain Lytechinus variegates sperm on time resulted in the eggs sitting in the solution for much longer than we would have liked. This experiment should be replicated without that delay for better results.
Sources
Longo, F.J. & Anderson, E. (1970), The Effects of Nicotine on Fertilization in the Sea Urchin, Arbacia Punctulata. The Journal of Cell Biology. 46:308-25
Jaffe, L.A. (1980), Electrical Polyspermy Block in Sea Urchins: Nicotine and Low Sodium Experiments, Develop., Growth and Differ., 22(3):503-7.
Jaffe, L.A. & Cross, N.L. (1986), Electrical Regulation of Sperm-Egg Fusion. Annual Review Physiology. 48:191-200.
