
The Effect of Saccharin on the Development of Sea Urchin Embryos
Erin O’Donnell, Melanie Hamilton and Steve Sarzynski
Millersville University
Objective
Determine if saccharine creates abnormalities during the gastrulation of sea urchin embryos.
Background
Many chemicals have been introduced during our lifetime. Constantine Fahlber first discovered saccharin in 1897, with the chemical structure being C7H5NO3S. In 1907 saccharin was used as a replacement for sugar in foods for diabetics. Since saccharin cannot be metabolized by the body for energy, it is classified as a non-caloric sweetener. By the 1960’s, it became used on a massive scale in diet soft drinks (Saccharin). Today saccharin is the foundation of many low calorie and sugar free products and it can be used in tabletop sweetener, baked goods, jams, canned fruit, candy, and dessert. It has become one of the most studied food ingredients of its time and although the overall evidence indicates saccharin is safe for human consumption, there has been controversy over its safety. This wariness towards saccharin comes from a study done on rats where the researchers found bladder tumors in some of the male subjects that were fed unusually high doses of sodium saccharin. There have been over 30 human studies completed that support saccharin’s safety at human levels of consumption.
There have also been studies done where saccharin was introduced to developing frog embryos. During the gastrulation process, embryos were developed in 1.5 mL of 10 g/L saccharine saccharin solution. Abnormal body flexure and abnormalities of the tail were observed in many of these embryos (Sakamoto et. al, 1992). However, another study conducted on Drosophila showed that after testing a range of possible tumor promoters on Drosophila embryos, saccharin was one of the two compounds that did not function as a tumor promoter. While various experiments have explored the effects of saccharin as a teratogen, its effect on embryonic development has not been studied to the same extent, which is the purpose of this study. In this experiment, sea urchin embryos were chosen as the experimental organism because of their sensitivity to environmental and chemical changes. Gaining information on the effect of saccharin on embryonic development could lead to further knowledge on how the sweetener could affect human developing embryos.
Materials:
male
and female gametes (Lytechinus variegates) saccharin
sodium 1g/L, 2g/L, 5g/L, 1 medium test tubes
10g/L, and 15g/L
sterile syringes and needles light
microscope
glass
depression slides digital
camera
petri dishes with covers (7)
glass
Pasteur pipets
artificial salt water (ASW)
Procedure
1) Place unfertilized eggs into 6 test tubes and wash 4 times with ASW.
2) A drop of concentrated sperm was placed in 4 mL of ASW.
3) Fertilize all the eggs in the tube with 1 to 2 drops of dilute sperm (note the time).
4) Allow the egg and sperm mixture to sit for 5 minutes and then add 10 mL of ASW to each test tube. Then transfer the fertilized eggs and 30 mL of ASW into seven glass dishes according to the appropriate temperature.
The following steps (5-10) should be performed simultaneously:
5) 1 of these dishes will be the control and it will be incubated at room temperature for the remainder of the experiment.
6) Add 1.5 ml of saccharin sodium (1g/L) to two of the dishes. Allow these dishes to incubate at room temperature for the remainder of the experiment.
7) Add 1.5 ml of saccharin sodium (2g/L) to two of the dishes. Allow these dishes to incubate at room temperature for the remainder of the experiment.
8) Add 1.5 ml of saccharin sodium (5g/L) to two of the dishes. Allow these dishes to incubate at room temperature for the remainder of the experiment.
9) Add 1.5 ml of saccharin sodium (10g/L) to two of the dishes. Allow these dishes to incubate at room temperature for the remainder of the experiment.
10) Add 1.5 ml of saccharin sodium (15g/L) to two of the dishes. Allow these dishes to incubate at room temperature for the remainder of the experiment.
11) Once these dishes have incubated for 24 hours, take pictures and determine if any abnormalities are present. Abnormalities may include changes to the shape of the embryo or the formation of the archenteron.
12) Repeat step 11 for 48 and 72 hours
Results & Discussion
The results of this experiment indicate that saccharin does indeed have negative developmental effects on sea urchin embryos. Figure 1 shows an embryo that served as the control by being developed in ASW. Upon examination it is evident that the embryo exhibits all of the normal phenotypes of a developing sea urchin embryo that is about twenty-four hours old. There is formation of the archenteron and secondary mesenchyme cells at the tip of the archenteron that will become the mouth of the embryo. The embryo is also very symmetrical and exhibits the proper shape that one would expect to see in a normally developing sea urchin embryo. In Figure 2, the embryo had received a saccharin treatment at a concentration of 1 g/L. While there is still clear formation of the archenteron, it is apparent that there may be the beginnings of slight malformations along the side of the archenteron. The embryo in Figure 3 had received saccharin at a concentration of 2 g/L and the effects are much more visible. The archenteron appears lumpy and malformed, while the lower sides of the embryo appear to be growing out slightly. Saccharin was used at a concentration of 5 g/L on the embryo shown in Figure 4. The embryo is clearly malformed, with more extreme changes than the embryo in Figure 3. Not only is the archenteron short, thick and lumpy, but the embryo itself has an awkward shape and a strange growth at the bottom of the embryo below the beginning of the archenteron that could be ectoderm. In Figure 5, the sea urchin embryo was treated with saccharin at a concentration of 10 g/L. The high concentration of saccharin clearly had an impact on the development of the embryo because there is not much that is visible. There is no archenteron present and the embryo appears to be developing two growths on each side. Figure 6 further proves the effects of large doses of saccharin. The embryo was treated with 15 g/L of saccharin, and similar to the embryo in Figure 5, it has no archenteron formation and appears to be very malformed. The only development that is visible in Figures 5 and 6 is the spicuoles that form from the primary mesenchyme cells.
The results of this experiment strongly indicate that at high concentrations, saccharin can have a significant effect on embryonic development. As the concentration of saccharin increased, the embryos exhibited more developmental abnormalities, until the embryos reached a point of having just calcified spicuoles and no gut formation. While the experimental embryos given concentrations of saccharin less than 10 g/L were able to have at least some rudimentary form of gut development, it is unknown whether or not those embryos could have survived to develop into normally functioning sea urchins. Although the FDA approved saccharin for human consumption, studies like this on other organisms indicate that more research may need to be done before a definitive answer can be given. It is important to take into consideration the fact that the National Toxicology Program keeps saccharin on its list of “anticipated carcinogens”, and so daily intake of the sweetener should be monitored. Also, while mild consumption of saccharin may be safe for humans, it is debatable as to whether expectant mothers could safely consume saccharin without endangering the unborn child. The effects of the sweetener on developing sea urchin embryos indicates that organisms undergoing development can experience dramatic detrimental changes when exposed to saccharin at mild to high levels. Indeed, other animal studies have also indicated that saccharin can behave as a tumor-promoter but there are no definitive answers as to why it seems to have this effect inconsistently depending on the type of organism being studied. The amount of saccharin intake likely has an effect on this, as it did in this experiment. While the basis for this study was based off of the work performed on frog embryos by Sakamoto, et al., this experiment on sea urchin embryos did not use a pure concentration of saccharin as the frog embryo study did. Future studies may want to investigate how sea urchin embryos respond to saccharin treatments that are not dilute, as the treatments in this experiment were.
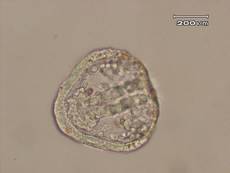
Figure 1. Sea urchin embryo treated with ASW. The embryos in the ASW treatment served as the controls.

Figure 2. Sea urchin embryo treated with saccharin at a concentration of 1 g/L. This embryo exhibited normal functional development, but the archenteron is not completely straight.
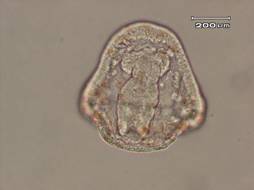
Figure 3. Sea urchin embryo treated with saccharin at a concentration of 2 g/L. This embryo is exhibiting growths on the outside of the structure and the archenteron is lumpy and malformed.
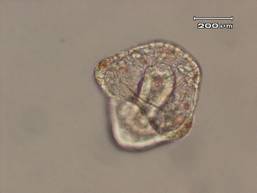
Figure 4. Sea urchin embryo treated with saccharin at a concentration of 5 g/L. This embryo exhibited many malformaties. The embryo itself was slightly squished and had a growth on one side. The embryo is not symmetrical and the archenteron is also formed off to one side.
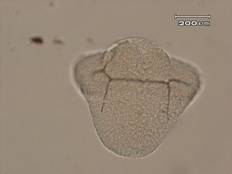
Figure 5. Sea urchin embryo treated with saccharin at a concentration of 10 g/L. This embryo did not complete gastrulation. There was spicuole formation, but no archenteron. There is also a large bulge at the bottom of the embryo that could be endoderm.
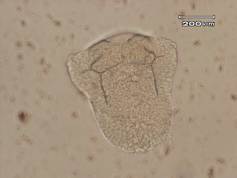
Figure 6. Sea urchin embryo treated with saccharin at a concentration of 15 g/L. This embryo also did not complete gastrualtion and only had spicuole formation.
References
Bournias-Vardiabasis, N., & Flores, J. C. (1986). Response of Drosophila Embryonic Cells to Tumor Promotor. Toxicology and Applied Pharmacology, 85, 196-206.
"Saccharin." Elmhurst College: Elmhurst, Illinois. 2003. 16 Mar. 2009 <http://www.elmhurst.edu/~chm/vchembook/549saccharin.html>.
Sakamoto, Michiko K., Shin Mima, and Takashi Tanimura. "An Assay System for Developmental Toxicity Using Embryos and Larvae of Xenopus laevis." AATEX 1 (1992): 172-77.