|
Effect of Ultraviolet Radiation on Fertilization
Capabilities of Lytechinus variegatusÕ Seamen
Rachael Curley, Zac Martin and Michael Mendez
Millersville University
Objective
To study the effects of ultraviolet
radiation (UV) emitted at 366 nm & 302 nm wavelengths on Lytechinus
variegatusÕ seamen and its ability to
fertilize or lack thereof after being exposed to UV at different time
intervals. If fertilization occurs, the long-term effects on the zygote will be
examined to determine if any developmental abnormalities occur.
Background
Lytechinus variegates, like most sea urchins, reproduces sexually by
uniting gametes (sperm & egg).
When conditions are ideal, sperm and eggs are released by the sea urchins
into the water column which is used for transportation of the gametes. Once fertilization occurs, mechanisms
are triggered to prevent
polyspermy. Na+ influx causes
a depolarization effect where the sperm are pushed away. The Ca++ that was stored in
the Endoplasmic Reticulum is now released across the egg, causing the cortical
granules to fuse, and release enzymes and hyalin. The enzymes digest the protein
bridges between the vitelline envelope and the eggÕs cell membrane. This fastens the vitelline envelope to
the egg plasma membrane. The enzymes
also destroy the sperm-binding sites and the fertilization envelope lifts away
from the zygote. This serves as
the permanent blockage for polyspermy (Gilbert).
In this experiment we plan to test the effect of ultraviolet radiation at 302 and 366 nm wavelengths on the sperm of L. variegates and their ability to fertilize. If they are capable of fertilization under these conditions, we will follow the embryo through development to observe any abnormalities and the frequency at which these abnormalities occur. Few studies have measured the effects of UV irradiation on eggs and embryos (Lu, 2005). According to Lu, the harmful effects of UV radiation may have a significant affect on sea urchins spawning in shallow waters. This may pose an environmental threat. Exposure to UVR has been shown to affect fertilization success, the timing of cleavage and the development time for embryos and larvae of the green sea urchin Strongylocentrotus droebachiensis (Adams and Shick, 2001; Lesser and Barry, 2003). Many of the embryos and larvae survive these exposures, but in the study by Lesser and Barry (2003) all developmental stages tested exhibited significant DNA damage, which was highly correlated with delays in cell division and developmental delays.
Theoretically, sperm should be more susceptible to UV
radiation due to (1) the lack of sunscreens such as mycosporine-like amino
acids to protect against UVR (Karentz et al. 1997; Adams et al. 2001); (2)
lower DNA repair ability (Donelly et al. 2000) and limited antioxidant
potential due to the small cytoplasmic volume (Aitken et al. 1998); (3) a greater
surface area to volume ratio, and (4) a high level of polyunsaturated fatty
acids in their cellular and intracellular membranes, making them particularly
prone to lipid peroxidation (Aitken et al. 1998). A significant decline in
motility of the sea urchin (Anthocidaris crassipina) sperm can occur in the
presence of UVB. This can lead to
a reduction in fertilization success (Lu et al. 2005). Fertilization and embryo development
should be delayed or stopped depending on the amount of UVR the embryo is
exposed to. The sperm of the sea
urchin Lytechinus variegates will be
exposed to UV radiation at two different wave lengths, 302 nm and 366 nm
(distance from peak to peak of the wave) and the success rate of these sperm
for fertilization will be observed based on the presence of the fertilization
envelope.
Materials
366 nm UV lamp
302 nm UV lamp
0.5 M KCl (potassium chloride)
Sea Urchins (L. variegatus)
Syringe + 22g needle
Artificial Salt Water (ASW)
2 x 1.5 inch stand
Glass Pasteur and Bulbs
3 x 250ml Beakers
4 Petri Dishes
25 medium test tubes
Test tube holder
Microscope
Depression Slides + Cover slips
Stop watch
Labeling tape
Experimental Procedure
1. Obtain 5ml of 0.5 M KCl in a syringe.
2. Inject the sea urchin in 3 different places in the soft muscle located around the mouth.
3. Turn the Sea Urchin upside down over a 250ml beaker filled with ASW. The sperm is milky white while the eggs can range from an off white color to orange in color. The male sperm should be moved to be inverted over a dry petri dish while females must be inverted over an ASW filled beaker.
4. Place ½ the sperm into a dry petri dish and the other ½
into a separate dry petri dish. Place on ice until ready for use. Dilute 1 drop (50µl) per 1ml of ASW for
use and split into two dry petri dishes.
5. Place the eggs into 3 medium test tubes and fill each tube 75% full
with Artificial Salt
Water (ASW).
6. After the eggs settle to the bottom, slowly decant nearly all of the ASW out of each tube
into a waste container. Fill the tubes again 75% full with ASW.
7. Repeat step 7 four times for each tube.
8. Using a glass pipette, transfer equal amounts of the
ASW-egg solution to 22 different
test tubes and place in a test tube holder. Separate the tubes into two groups of 11.
One set is for the 302 nm wavelength and the other is for the 366 nm wavelength. In
each group, label a control and 1-10min tubes.
9. To set up the 306nm light source, place two blocks on the table and place the light on
top of them leaving the center of the light source unblocked. The blocks should
make the light source an inch and a half above the surface of the table.
10. Take one of the Petri dishes containing the diluted sperm. Pipette a small amount of
sperm into one of the test tubes labeled control. Turn the 306nm light source on
and place the sperm under it. Start the timer.
11. Starting at 1 min, pipette a small amount of sperm out of the Petri
dish (while still
under the UV light source) and place into tube labeled 1 min. Repeat this step every
minute for 10 minutes placing the appropriate timed sperm with the matching tube.
12. Repeat steps 10-12 with the 366 nm light source.
13. For each UV treated sperm, observe them under the microscope 10 minutes after they
are combined with the eggs. The first ten eggs seen should be documented on whether
or not they have a fertilization envelope present.
Results
The sperm at 302 nm wavelength UV light had a dramatic effect on the Lytechinus variegatus spermÕs ability to fertilize. Two trials were conducted using the 302 nm wavelength. During the first trial, virtually no fertilization occurred once the sperm had been exposed for 4 minutes (figure 1). The second time exposure of sperm to 302nm UV was tested, it took 2 minutes longer to block fertilization from occuring after exposure (figure 2). It is extremely hard to replicate the conditions so exactly that the exact same minute is seen in both trials. The second trial used more time intervals to get a more exact minute of when fertilization no longer takes place. In contrast, when the sperm was exposed to the 366 nm UV light, there seemed to be no effect on the sperms ability to fertilize the L. variegatesÕ eggs even after 10 minutes of exposure(see figure 3).
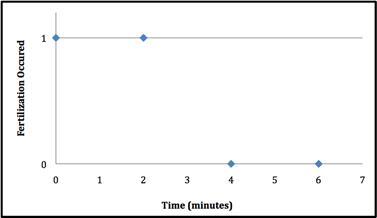
Figure 1: Exposure to UV at 302 nm destroys the ability of sea urchin sperm to fertilize eggs after 4 mintues. The time of exposure to 302 nm UV light vs. whether or not fertilization took place. If fertilization did occur itÕs notated by a 1. If fertilization did not occur itÕs notated by a 0.
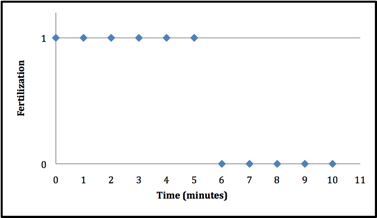
Figure 2: Exposure to UV at 302 nm destroys the ability of sea urchin sperm to fertilize eggs after 6 mintues. The time of exposure to 302 nm UV light vs. whether or not fertilization took place. The sea urchin sperm was exposed to 302nm UV light for an increment of time and then placed with sea urchin eggs. Fertilization was documented on the presence of a fertilization envelope 10min after first combining them. If fertilization did occur itÕs notated by a 1. If fertilization did not occur itÕs notated by a 0. It took 20% longer for the effects of UV exposure to be seen on fertilization.
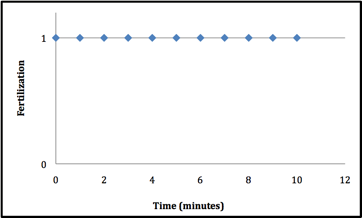
Figure 3: Exposure of the sperm to 366 nm UV light did not have an effect on the fertilization capabilities. If fertilization did occur itÕs notated by a 1. Fertilization was not inhibited during the 10 minute duration of 366 nm UV light exposure.
Discussion
The first experiment using 302 nm UV light showed that the 302 nm UV light did have an effect on the fertilization capabilities of the sperm after 4 minutes of exposure. The second experiment using the same wavelength UV light did show impedance of the fertilization capabilities of the sperm, just under longer exposure. In contrast to the first experiment, the sperm became unable to fertilize the eggs after 6 minutes of exposure. The 366 nm UV light was expected to also inhibit the sperms ability to undergo fertilization, but did not. There are two reasons why the same results for the 302 nm UV light were not obtained. (1) the concentration of sperm was much greater and (2) due to the high amount of sperm there was more ASW in the Petri dish which could have lessened the affects of the UV light at deeper depths. The 366 nm UV light did not have as great of an effect on the sperm as the 302 nm UV light because it is less energetic. The UV does less damage to the cell and does not hinder the sperms ability to fertilize eggs as supported by this data.
References
Adams
NL, Shick JM, Dunlap WC (2001) Selective accumulation of mycosporine-like amino
acids in ovaries of the green sea urchin Strongylocentrotus
droebachiensis is not affected by ultraviolet
radiation. Mar Biol 138:281–294
Aitken
RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. Relative
impact of
oxidative stress on the functional competence and genomic
integrity of human spermatozoa. Biol Reprod 1998; 59:1037–1046.
Donnelly
ET, McClure N, Lewis SE (2000) Glutathione and hypotaurine in vitro: effects on
human sperm motility, DNA integrity and production of
reactive oxygen species. Mutagenesis 15, 61–68
Gilbert, Scott F. Developmental
Biology.
Sunderland, Mass.: Sinauer Associates, 2006. Print.
Karentz
D, Dunlap WC, Bosch I (1997) Temporal and spatial occurrence of UV-absorbing
mycosporine-like amino acids in tissues of the Antarctic
sea urchin Sterechinus neumayeri during springtime ozone-depletion. Mar Biol
129:343–353
Lesser
MP, Kruse VA, Barry TM (2003) Exposure to ultraviolet radiation causes
apoptosis in developing sea urchin embryos. J. Exp. Biol.
206, 4097–4103.
Lu X. Y, R. S. S. Wu (2005) UV induces reactive oxygen
species, damages sperm, and
impairs fertilization in the sea
urchin Anthocidaris crassispina. Mar
Biol 148: 51–57
