|
Histochemical staining of sea
urchin embryos for alkaline phosphatase (AP) enzyme
activity
1.
Obtain embryo samples, tube of AP
substrate buffer and tube of
phosphate buffered saline (PBS)
for each group. Allow embryos to settle. Carefully remove
supernatant.
2.
Resuspend in 0.5 ml AP substrate buffer. Allow embryos to
settle for 10 min. Remove excess buffer.
3. Add
100 ul AP substrate to tubes. Check for staining after 5
minutes by transferring a small sample to depression slide
and observing on 4X or by observing tube of embryos using
dissecting microscope. Be careful not to get AP substrate
on your hands (wear gloves) or on your microscope . Do
not leave light turned on between observations. To stop the
reaction, return embryos to the tube and add 0.5 ml
PBS.
4.
Allow embryos to settle for 10 minutes. Remove buffer to
about 100 ul, return to depression slides and observe. Look
for evidence of morphogenesis (archenteron invagination) and
tissue differentiation (gut alkaline phosphatase activity
and spicule formation). Document your observations by
capturing images of your stained embryos.
Alkaline Phosphatase substrate:
The substrate is a combination of
nitro blue tetrazolium (NBT) and
5-bromo-4-chloro-3-indolyl-phosphate (BCIP).
Make fresh from stocks:
22.5 ul NBT (100 mg/ml, Roche 11
383 213 001)
16.5 ul BCIP ( 50 mg/ml, Roche 1 383 221)
bring to 5 ml with AP substrate buffer.
An alternate is ready-to-use Western
Blue stabilized substrate (Promega)
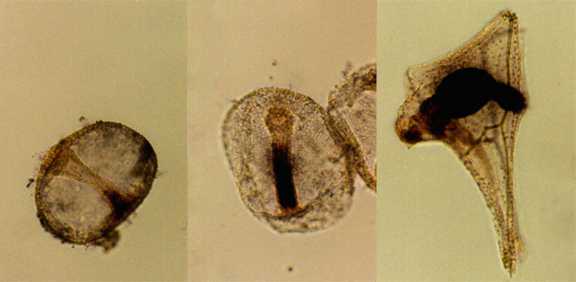
Alkaline phosphatase activity in L. pictus gastrulae
(left and center) and pluteus larva (right).
|