|
Procedure
(adapted from Hamburger, 1960)
Week 1
1) Sterilize host embryo
shell (10-day old embryo) with 70% ethanol. Candle
the egg to locate blood vessels near the blunt end
of egg by holding egg against light (fig. 1). At
the blunt end of the egg, create a hole
approximately 1.5 cm in diameter with forceps.
Remove shell and shell membrane, keeping the CAM
(membrane that lies beneath) intact. After locating
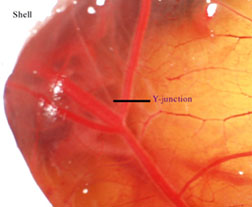
a large Y-junction of blood vessels (fig. 2), seal
the hole with scotch tape (fig. 3) and store egg in
37 degree Celcius incubator.
|
|
|

|
|
Figure 1.
Candling procedure to locate blood vessels
in egg.
|
Figure 2.
Y-junction on chorioallantoic
membrane.
|
Figure 3. Hole
punctured on blunt end of egg covered with
Scotch tape.
|
2) Sterilize donor embryo
(5 day-old) shell with 70% ethanol. Open blunt end
of shell with forceps and transfer the embryo from
egg into dish of Ringer's solution. Remove and peel
away surrounding membranes from the embryo and
photograph the embryo using digital microscopy.
Excise limbs (2 forelimbs and 2 hindlimbs) from
embryo using fine forceps. Include extra flap of
cells from flank if possible to help position graft
onto CAM.
3) Transfer one limb
graft into each host embryo. Gently position graft
over Y-junction of blood vessels and reseal egg
with scotch tape to prevent infection. Incubate at
37 degrees Celcius for 7 days.
4) Isolate 5-day old
donor embryo as a control for cartilage development
at the 5-day stage. Fix in 4% PFA in plastic tube
and gently agitate overnight.
5) Set aside untampered
5-day old donor embryo (shell intact) in 37 degree
Celcius incubator for 7 days to serve as a control
for normal cartilage development in intact
embryo.
6) Repeat entire
procedure using 7-day old donor embryo.
Week 2
7) Candle hosts to
determine which are viable. Remove tape and dissect
graft from CAM. Excise limbs from intact donor
controls from step 5. Place limbs in individual
glass vials. Immerse in 5% trichloroacetic acid
(TCA) for 1 hour at room temperature to fix
sample.
8) Wash 3 times with
enough phosphate buffered saline (PBS) to cover the
limb or embryo in order to remove TCA and return to
proper pH and salt concentration.
9) Immerse in Alcian
green stain for cartilage overnight.
10) Wash 3 times with 70%
ethanol to dehydrate sample. Repeat with one wash
each in 85%, 95%, and 100% ethanol to remove
remaining water from sample.
11) Transfer to methyl
salicylate to clear limbs and further dehydrate the
sample for better visualization. Allow sample to
equilibrate overnight.
12) Photograph limbs
under microscope linked to LCD digital camera.
Compare development of limb structures and
formation of cartilage/bone in the graft limb and
in the controls. Try to identify major bones in
graft and control limbs and observe any differences
in appearance.
|





![]()
![]()
![]()